Original Blog Articles from TruMed Edmonton

Dr. Eric
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS.
This Blog is devoted to exploring Natural medicine options and Multiple Sclerosis.
SinaCurcumin & MSSinaCurcumin is a "nano-curcumin" with 80 mg of curcumin in "nano-micelles" for enhanced absorption. In a 2018 study by Dolati, 25 RRMS patients received 80mg of SinaCurcumin daily for 6 months compared to a placebo group. Blood samples were taken at baseline and after 6 months. All patients were also receiving Interferon Beta-1a. The proportion of Treg cells in the nanocurcumin treated group significantly increased which have a crucial role in the maintenance of self-tolerance. Treg cells can suppress inflammation and immune responses via several mechanisms including the secretion of IL-10 or TGF-β. Furthermore, nanocurcumin administration caused a significant increase in TGF-β and IL-10. Lastly, curcumin appeared to also improve the EDSS for the patients as well. Another study by Dolati, also in 2018, showed that nanocurcumin is able to restore the expression pattern of dysregulated MicroRNAs (miRNAs - a type of RNA) in MS patients. MicroRNAs are differentially expressed between MS disease subtypes and healthy individuals. Initially, 13 miRNAs were found to be upregulated and 16 miRNAs are downregulated from patients with RRMS - the expression of 11 of which were restored with nanocurcumin administration. We've safely incorporated Curcumin into our autoimmune protocols at our Edmonton office. Curcumin is thought to traverse the blood brain barrier and historically has been found to positively influence inflammatory cytokines and the animal model of MS. Our office provides Edmonton with intravenous (IV) curcumin treatment. |
NAD+ and MSNAD+ has emerged as a major player in healthy aging and in particular has demonstrated broad applicability neurodegenerative diseases. Reduced NAD+ levels contribute to most, if not all, of the hallmarks of brain aging. In 2013, Braidy et. al looked at NAD+ levels in RRMS, PPMS and SPMS. NAD+ and NADH levels were measured in the serum from 209 patients with relapsing remitting MS (RRMS), 136 with secondary progressive MS (SPMS), 51 with primary progressive MS (PPMS), and 99 healthy controls. Serum NAD+ levels declined by at least 50% in patients with MS compared to controls. Within the MS sub-groups NAD+ levels were markedly higher in RRMS compared to PPMS and SPMS. Higher levels of NADH and a lower NAD+/NADH ratios were observed in MS patients compared to controls. impaired mitochondrial respiratory function, results in an elevated NADH:NAD+ ratio thereby inducing more free radical production via Fenton chemistry, which then activates PARP-1 which utilizes NAD+ as the substrate and may cause NAD+ depletion. Nicotinic acid (niacin) appears to be the preferred substrate for NAD+ synthesis when applied in high concentrations, and generates greater levels of NAD+ per mole than nicotinamide (niacinamide) or tryptophan by 200 and 500 fold respectively. Interestingly, in 2006 Kaneko showed niacinamide (acting as a NAD+) precursor "profoundly prevents the degeneration of demyelinated axons" in the animal model of MS. Based off the available data, we frequently try administration of IV NAD+ in our Edmonton MS patients for symptomatic relief. However, it is clear that maintaining increased NAD levels can be beneficial in reducing the neurodegeneration inherent to the disease. |
Umbilical Cord Stem Cells & MSUmbilical Stem Cells and MSJune 2020 Mesenchymal stem cells (MSC) derived from bone marrow, adipose, or other sources can exert inhibitory Twenty enrolled subjects received 140× 106 UCMSC intravenously over the course of seven visits (20× 106 UCMSC/day) were enrolled in this study by Riordan et al. It is of interest that Riordan is the son of Riordan that popularized IV Vitamin C Therapy. Follow-up visits, scheduled at 1, 3 month and 1 year post-treatment with various standard efficacy parameters assessed as well as baseline and 1 year post treatment MRI. There were no Mean age of enrollees was 41.15 years and 60% were female with mean disease duration of 7.7 years. Fifteen subjects (75%) had a diagnosis of relapsing–remitting MS, four (20%) with primary progressive MS, and one (5%) with secondary progressive. Eleven subjects (55%) required ambulation assistance (wheelchair, walker, or cane) at baseline. Nineteen subjects were followed for 1 year. there were no reported serious adverse events. At baseline, EDSS
At 1 month and 1 year, more than half of the subjects reported their condition as better or the same on all scale scores. No common themes were detected regarding number of lesions or disappearance of lesions. The results of this study are by no means miraculous but it's promising to see some appreciable benefit even when stem cells are not administered into the central nervous system itself. It would be of interest to see if "piggybacking" Hyperbaric oxygen therapy or Hyperbaric pretreatment would enhance the effects. |
Ketogenic Diet & MS MicrobiomeKetogenic Diet and the MicrobiomeMay 2020 Blog post Fourteen healthy volunteers and 25 patients with relapsing-remitting multiple sclerosis (RRMS) received a ketogenic diet for 6 months. The ketogenic diet was designed to achieve a modest ketosis (≥0.5mmol/L in the urine). Average daily intake of < 50 g carbohydrates, >160 g fat, and < 100 g protein was recommended. Ten of the MS patients who were recruited for the ketogenic diet were randomly selected for accompanying microbiome investigations with samples collected at baseline, week 2, 12, and after 6 months. Three bacteria detected mainly Roseburia, Bacteroides and Faecalibacterium prausnitzii were always present in healthy human controls and MS patients and contributed to about half of the colonic microbiome in each subject - these groups were called essential bacteria. The concentrations of most investigated groups in MS were decreased. Both concentrations and biodiversity of numerically substantial bacterial groups were markedly reduced in MS (the diversity of all substantial groups in MS patients was reduced by 36%). Essential bacteria were most decreased (32%), individual substantial bacteria less depleted, while pioneer bacteria were nearly unchanged (Pioneer bacteria are usually found in low concentration in healthy adults). Except for Akkermansia, all groups of bacteria demonstrated consistently marked decreases, leading to a reduction of the total bacterial concentrations of the substantial bacteria at week 2. The total bacterial concentrations in MS patients started to increase at week 12, reaching values typical for healthy controls at week 24. The concentrations of pioneer bacterial groups (found in newborns) fell and remained low, the concentrations of Akkermansia increased initially but then declined during the ketogenic diet. After 6 months on the ketogenic diet, the sum concentrations of the substantial microbial groups in MS patients increased significantly when compared to the period prior to intervention (83 vs. 65 × 109 bacteria/ml) and became indistinguishable from the healthy group (83 vs. 85.4 × 109 bacteria/ml.) Ketogenic diets might be not only an appropriate intervention to increase energy production in the nervous system but may aid in restructuring of the gut microbiome which then may modulate the immune system. Our Naturopathic doctors work with Ketogenic diets with our MS patients. |
MS & MelatoninHigh Dose Melatonin & MSPosted April 2020 Melatonin is synthesized in the pineal gland and all mitochondria containing cells to some degree, including Melatonin has been shown to have paradoxical proinflammatory properties. Melatonin exhibits pro-inflammatory effects by upregulation of major histo compatibilitycomplex-II (MHC-II) expression and increase in Th1-related cytokines through activation of surface melatonin receptor on antigen presenting cells. On the other side, melatonin has been shown to have impact neuroprotective and anti-oxidant properties likely via inductions of anti-oxidant enzymes. In one 6 month study with 36 RRMS patients, 18 received 25 mg/d of melatonin capsules and 18 received Decreases were seen: 18% for TNF-α, 35% for IL-1β, 35% for IL-6, 40% for lipoperoxides, and 24% for nitric oxide catabolites (NOC) levels compared with placebo group (oxidative stress markers). During the course of the clinical trial, one patient in the placebo group presented 3 relapses in a 2 month period (with a diagnosis by the treating physician of secondary progressive multiple sclerosis). No patients in melatonin group presented with relapses. No significant differences between melatonin and placebo group in the clinical efficacy outcome (EDSS score and depression level) at 6 months of follow-up . Previous studies showed decreased serum concentrations of melatonin and its metabolites in patients with MS A different trial in 2013, 16 SPMS patients supplemented with 10mg melatonin for 30 days assessed super oxide dismutase, (SOD), glutathione peroxidase (GPx) - antioxidant enzymes and malondialdehyde (MDA) a marker of lipid peroxidation because imbalances in oxidative damage and oxidatiive stress are thought to be drivers of SPMS neurodegeneration. When SPMS patients were treated with melatonin the SOD and GPx activities distinctly increased and CAT activity slightly increased. Melatonin caused a decrease of MDA activity in both patients and control groups. An observed improvement in functional status of SPMS patients assessed by EDSS and MSIS scales but not statistically significant indicating that in the short term these doses are not detrimental in SPMS.
So in summary, high (25mg) and low dose (3mg) melatonin appears safe in RRMS and whereas moderate dose (10mg) appears safe, at least in the short term in stable SPMS. I personally still exercise caution since melatonin has long history of known TH1 enhancing activity hence its use in alternative cancer care but these results are encouraging especially because of well known sleep disturbances in the MS population as well as a lack of robust options for preventing neurodegeneration. |
HERV & MSHuman Endogenous Retroviruses & MSPosted March 2020 The most consistent studies exist for the interaction of Epstein–Barr virus (EBV), Human Herpes Virus 6 (HHV-6) and two members of the W family of human endogenous retroviruses (HERV): MS-associated retrovirus (MSRV) and ERVW-1. The HERVs are intermediate between exogenous viruses and genes, as they are the remnants of ancient infections by regular retroviruses. MSRVenv and Syncytin-1 are encoded by MSRV and ERVW-1 respectively and are thought to be pro-inflammatory and superantigenic. In a 2006 paper, autopsied brain tissues from MS patients and controls and peripheral blood mononuclear cells (PBMCs) were analysed. Early post-mortem brain tissue from five MS subjects with a clinical diagnosis of chronic progressive MS were studied with 3 of 5 cases with predominantly chronic-active lesions. Controls consisted of four normal brain controls (NBCs) and six patients with other neurological diseases (OND) and four patients with grade IV astrocytoma. Blood samples from 35 patients with active MS and 14 healthy donors were analyzed. Two different transcripts were analyzed for each virus [MSRV/HERV-W] env and pol, encoding envelope and reverse transcriptase and HHV-6 U94/rep, which maintains the latent state, and DNA-pol, indicative of actively replicating virus. Accumulation of MSRV/HERV-W-specific RNAs was significantly greater in MS brains than in controls. No HHV-6-specific RNAs were detected in brains of MS patients. MSRV/HERV-W env transcripts accumulated similarly in brain samples from NBCs and OND controls, without significant differences between white and grey matter. In brain samples from MS patients, however, the accumulation of MSRV/HERV-W transcripts was increased by 20- to 25-fold At the PBMC level, all MS patients expressed MSRV/HERV-W env at higher copy numbers than did controls. The authors do note however that in contrast, a higher prevalence in the MS brain was found for HHV-6 It's thought that herpesviruses might activate HERV expression in patients, as MSRV expression can be Interestingly, when MS patients undergo successful therapies, the expression of MSRV diminishes accordingly. Gilenya, Copaxone, Tysabri and Avonex all are associated with MSRV drops. One drug treatment: GNbAC1 - a monoclonal antibody directed against the envelope protein of a HERV has been studied in humans - anticipated immunomodulatory effects were not observed in the trials of this medication.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: Ther Adv Neurol Disord. 2019 Mar 7;12:1756286419833574. doi: 10.1177/1756286419833574. eCollection 2019.
The monoclonal antibody GNbAC1: targeting human endogenous retroviruses in multiple sclerosis. Diebold M1, Derfuss T2. J Gen Virol. 2007 Jan;88(Pt 1):264-74.
Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not Human herpesvirus 6.
Mameli G1, Astone V, Arru G, Marconi S, Lovato L, Serra C, Sotgiu S, Bonetti B, Dolei A. |
Probiotics & MS
Probiotics & MSPosted on Dec 2019 In a 2018 trial, nine MS patients and thirteen controls were a sachet of VSL #3 (now called Visbiome in Canada) twice-daily for two months. Blood and stool specimens were collected at baseline, after completion of the 2-month treatment, and 3 months after discontinuation of therapy. VSL#3 is a high dose (450 billion) probiotic that has been studies in Ulcerative Colitis, Pouchitis and IBS. Administration increased the abundance of bacteria known to be depleted in MS such as Lactobacillus and increase in the relative abundance of family Veillonellaceae and genus Collinsela that are depleted in the gut of MS patients and decreased the abundance of taxa previously associated with dysbiosis in MS, including Akkermansia (considered a TH1 inducer), Dorea and Blautia. Decreased methane metabolism was also found (which is good because increased methane producing species like methanobrevibacter smithii have been found in MS). At the immune level, probiotic administration induced an anti-inflammatory peripheral immune response characterized by decreased frequency of inflammatory immune cells. Unfortunately, the overall microbial community structure shifted back to baseline level following discontinuation of Overall, this indicated that Visbiome/VSL#3 is a suitable and effective probiotic to use in MS patients. It is a bit cost prohibitive however as it typically costs around $150 per month (Canadian dollars). Select references: Ann Neurol. 2018 Jun;83(6):1147-1161. doi: 10.1002/ana.25244. Epub 2018 Jun 8.
A probiotic modulates the microbiome and immunity in multiple sclerosis. Tankou SK1,2, Regev K1, Healy BC1, Tjon E1,2, Laghi L3, Cox LM1,2, Kivisäkk P1,2, Pierre IV1,2, Hrishikesh L1, Gandhi R1,2, Cook S1, Glanz B1, Stankiewicz J1, Weiner HL1,2. |
Baicalein & MSBaikal Skullcap & MSPosted on February 8th 2020 Baicalein is isolated from the roots of Scutellaria baicalensis (Baikal Skullcap) which has has been shown to exert anti-inflammatory, antioxidant effects and importantly anti-viral and neuroprotective effects. Neuroinflammation, including microglial activation and enhanced pro-inflammatory cytokine production are key components of the pathological process of MS. The cuprizone model is a mouse model of MS. Hashimoto et al., administrated Baicalein to mice in the cuprizone MS model. Demyelination coincides with microglial and astrocytic activation in the cuprizone model. Microglia are known to develop functional phenotypes of pro-inflammatory (M1) and alternative (M2) activations. M1 microglia are characterized to release pro-inflammatory cytokines, such as TNFα and IL-1β. In our results, Baicalein treatment attenuated the gene expressions of TNFα and IL-1β, Baicalein suppressed the demyelination, reduced weight loss & motor dysfunction. Baicalein treatment effectively attenuated cuprizone-induced microglial and astrocytic activation (resident immune cells in the brain that are involved in demyelination in RRMS AND SP & PPMS). Cuprizone exposure for 6 weeks increased the mRNA expression of the pro-inflammatory cytokines, TNFα and IL-β which was attenuated by Baicalein treatment (suggesting that Baicalein suppressed microglial M1 pro-inflammatory activation) as well as iNOS (iNOS-derived NO production via NF-κB inhibition in inflammatory conditions). The authors remark "Our results indicated that Baicalein may be beneficial for protecting oligodendrocytes and neuronal axons from an unfavorable microenvironment caused by excessive microglial activation under neuroinflammatory conditions." Data exists that Baicalein "has prophylaxis and therapeutical effect on infection caused by HHV-6," which makes it an amazing candidate for those with Viral infections and MS! Baikal skullcap is pretty much the best.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: Brain Res Bull. 2017 Oct;135:47-52. doi: 10.1016/j.brainresbull.2017.09.007. Epub 2017 Sep 15.
The flavonoid Baicalein attenuates cuprizone-induced demyelination via suppression of neuroinflammation.
Hashimoto M1, Yamamoto S1, Iwasa K1, Yamashina K1, Ishikawa M1, Maruyama K1, Bosetti F2, Yoshikawa K3.
|
Low Dose Naltrexone and MSLDN (low dose naltrexone)Posted on November 13 2016 Naltrexone at its full dose was approved by FDA in 1984 for the treatment of opioid addiction. Bernard Bihari, MD, discovered the effects of Naltrexone, in low doses, in humans in the 1980s. Low-dose naltrexone (LDN) has been demonstrated to reduce symptom severity in conditions such as fibromyalgia, I reviewed the journal article "Low Dose Naltrexone for Treatment of Multiple Sclerosis: A Retrospective Chart Review of Safety and Tolerability" published in 2015. This article analyzed the medical records of 215 MS patients, aged 18 to 65 years, seen in an MS clinic for a 7-year period which is an excellent resource regarding long term safety and efficacy. The safety, tolerability, and effectiveness of LDN (at doses of 3.5mg) on fatigue was analyzed. Seventy seven percent (n = 166) of patients taking LDN for any period of time did not report any side effects. Nearly 60% (n = 128) of patients receiving LDN for any period of time reported a reduction in fatigue with LDN therapy More importantly, 130 patients (60%) stated that LDN stabilized or improved their disease and 75% of the patients reported improved or stabilized quality of life. Only nine patients reported that LDN reduced the quality of life, and 8% of the patients had the perception that their disease increased while on LDN. Given the waxing and waning state of MS it is unclear if these declinations could truly be attributed to LDN. LDN is a slow acting treatment and it usually takes 3-4 months to work in its full capacity. One may pair LDN with anti-inflammatory treatments while we're waiting for its action to start working. Because of its action against opioids, it can negate medications like morphine, codeine, hydromorphone and oxycontin which usually are not a concern for most MS patients but we do run into issues with our cancer patients dealing with pain. It's been observed that LDN in it's full dose of 4.5mg may increase spasticity in MS patients therefore either reduced doses are given (3 - 3.5mg) or patients can use full dose LDN (4.5mg) and add anti-spasticity agents like CBD (see my CBD post!) Prior to the review I discussed, LDN had been studied in a 17 week study in MS patients and the results suggested an improved quality of life but the trial was clearly too short to see impact on more serious parameters like relapse frequency. "75% of the patients reported improved or stabilized quality of life."
I propose the addition of LDN to MS patients if they are on an immunomodulatory treatment but still actively relapse, or if they are not on active medication and still relapse and require help with symptoms such as fatigue. Because it does take a few months to fully work, I suggest using anti-inflammatory treatments such as oral curcumin, boswellia or GLA in the first few months of LDN use. It may be advantageous to combine LDN with High dose thiamine (Vitamin B1).
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: J Clin Psychopharmacol. 2015 Oct;35(5):609-11. Low Dose Naltrexone for Treatment of Multiple Sclerosis: A Retrospective Chart Review of Safety and Tolerability. Turel AP1, Oh KH, Zagon IS, McLaughlin PJ.
Ellis, Wendy (2015). Low Dose Naltrexone in the treatment of Autoimmune Disease.
|
Lemtrada and MSLemtrada and MS
Posted On December 19 2017 Alemtuzumab (Lemtrada) is among the most potent currently available drugs for disease modification in MS I reviewed of "Summary of Alemtuzumab in the treatment of Multiple Sclerosis. . ." a 2016 Journal Article. Lemtrada is potent: shortly after receiving Lemtrada the blood is virtually devoid of lymphocytes (type of immune cell). This medication is in my opinion the closest MS medication that approximates a Stem Cell transplant (AHSCT - autologous stem cell transplant - where the immune system is destroyed and "rebooted") In fact, some stem cell regimines use Lemtrada as part of the treatment regimine (See Burt et al. 2015) T Lymphocytes, repopulate slowly after Lemtrada reaching normal levels after ~2–3 years and pretreatment levels only after ~5 years Three main studies are usually referenced for discussing Lemtrada efficacy: CAMMS223, CARE-MS-1 and CARE-MS-II Across the three studies, alemtuzumab significantly reduced relapse rates relative to high dose IFN beta-1a. By 69% and 55% in the first-line treated patients (CAMMS223 and CARE-MS-I) and 49% in the second-line treated patients (CARE-MS-II) The NEDA “no evidence of disease activity” was: CARE-MS I: alemtuzumab 39%, IFN 27%; CARE-MS II: alemtuzumab 32%, IFN 14% The most disconcerting risk attributable to alemtuzumab-treatment is development of secondary autoimmunity. Almost half of the patients developed secondary autoimmunity over a follow-up of up to 10 years (incidence peak in the 3rd year). The best responders were suggested to be young patients with active disease and rather short disease duration (<10 years="" --10--=""> In the CAMMS223 10 year follow up a very low annual relapse rate was maintained (.07) "The best responders were young patients with active disease"
It is in my opinion that Alemtuzumab be considered as a therapy in all MS patients hat continue to relapse despite current treatment as it appears to be the closest therapy to AHSCT. Lemtrada infusions are immunosuppressive, and are given simultaneously with steroid infusions which are also immunosuppressive. Therefore, it may be beneficial to screen for viral, bacterial and fungal infections prior to the first set of infusions for infections that have been found to be related to MS progression. As their activity may increase during these immunosupressive treatments which might propagate disease in select individuals. Please see MS and Viruses Post 1 and Post 2
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: |
Lemtrada and MS Post 2Lemtrada Post 2
Posted On January 28 2017 As mentioned in Post 1, Lemtrada or Alemtuzumab is one of the most aggressive and efficacious MS medications currently available. The mechanism of action is essentially a soft reset on the immune system similar to a stem cell transplant (non-myeloablative - for those that enjoy medical jargon!) I Reviewed "Improvement in disability after alemtuzumab treatment. . . " a 2010 Journal Article. In the CAMMS223 trial, disability improved after alemtuzumab (Lemtrada) but not following interferon Beta 1a In the 10 year follow up of CAMMS223, 78% had stable or greater than 1-point improved EDSS (a disability scale from 1 to 10). The mechanism behind this stable or improved functional status appears to be that immune cells (T cells) appeared to produce increased concentrations of brain-derived neurotrophic factor (BDNF) BDNF enhances oligodendrocyte precursor cell survival and maturation. Oligodendrocytes create myelin sheath in the CNS and therefore Lemtrada appears to improve myelination in addition to it's reset capacity on the immune system and therefore subsequent reduction in relapse frequency. Therefore, it appears that in a subset of MS patients on Lemtrada there is an improvement in disability parameters. Apparently, this is hastened not only from a reduction in inflammation but by increased BDNF released from T cells "Lemtrada appears to increase BDNF"
It is in my opinion that Alemtuzumab/Lemtrada be considered as a therapy in all MS patients that continue to relapse despite current treatment as it appears to be the closest therapy to AHSCT (autologous stem cell transplant). It may be advantageous to use this treatment as a first line treatment to avoid any damage from the disease process rather than wait for the faliure of other therapies which is the current standard of practice that has left hundreds of thousands disabled. As mentioned in Lemtrada Post 1 it may be advantageous to test for bacterial, viral and fungal infections prior to administration of Lemtrada as these infections may thrive in the immune suppressed state induced by Lemtrada and can potentially perpetuate further disease. For further information on MS and Infections see MS and Viruses Post 1.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: Brain. 2010 Aug;133(Pt 8):2232-47. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Jones JL1, Anderson JM, Phuah CL, Fox EJ, Selmaj K, Margolin D, Lake SL, Palmer J, Thompson SJ, Wilkins A, Webber DJ, Compston DA, Coles AJ.
Drug Des Devel Ther. 2016; 10: 3379–3386. Alemtuzumab in the treatment of multiple sclerosis: patient selection and special considerations Jan Dörr and Karl Baum |
MS Fatigue - Thiamine
MS Fatigue Treatment
Posted On December 31 2016 In 2013 a Journal Article called "High dose THIAMINE improves fatigue in Multiple Sclerosis" was published. Thiamine is vitamin B1. Fifteen patients, nine women and six men with relapsing remitting MS were studied. High-dose thiamine therapy consisted of 600–1 500 mg/day orally or 100 mg once a week IV The evaluation of the fatigue was measured 20 days after the beginning B1. Improvement of the fatigue was shown in 14 patients out of 15 (93.3% of the cases) Improvement was within hours from the first IV administration or within 2–3 days after the beginning of the oral therapy Fatigue improved by an average of 41% A check-up after 18 months of treatment did not show any decrease of the efficacy of the therapy. Many studies show that administration of a form of B1 called benfotiamine leads to higher thiamine blood concentrations than administration of just thiamine. Benfotiamine strongly increases thiamine levels in blood but it has no significant effect in the brain. Sulbutiamine, is a fat-soluble B1 derivative, that increases thiamine in the brain. Therefore I propose using Sulbutiamine, partially, if not wholly in MS patients Doses of up to 600mg of Sulbutiamine have been used in Chronic Fatigue. Doses of up to 300mg daily of Benfotiamine have been used in Diabetes. "Fatigue improved by an average of 41%"
I propose a mix of the two, with 200-300mg of Benfotiamine combined wih 200-400mg of Sulbutiamine in MS fatigue. Intramuscular (IM) and Intravenous (IV) Thiamine is an option as well but this may not achieve the same brain levels as Sulbutiamine however, on the other hand, patients clearly responded well to IV thiamine in this trial. Our clinic focuses on IV Vitamin therapies and treatments in Edmonton.
As with any other patient experiencing fatigue, we need to always rule out other potential reasons: most commonly hormonal issues rather than assuming all of a patient's woes are due solely to the MS. MS fatigue can be improved not only by addressing nutrition as we discussed above but also by looking at a patient's hormone levels and their global health instead of focusing solely on the MS. I would urge MS patients to have their thyroid and adrenal glands evaluated by a functional medical doctor or a naturopathic doctor via comprehensive blood testing and salivary testing respectively in addition to nutritional treatments like B12 and B1. The thyroid and adrenals are by far the most common causes of fatigue I see at my clinic in Edmonton.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: BMJ Case Rep. 2013 Jul 16;2013. High dose thiamine improves fatigue in multiple sclerosis.
|
Artesunate and MS
MS & Artesunate
Posted on January 26 2017 Artesunate is a wormwood extract that can be taken orally or injected. Our Naturopathic Clinic has substantial experience with wormwood derivatives. I Reviewed the 2016 Journal Article 'Artesunate Amerliorates Experimental Autoimmune Encephalomyelitis by Inhibiting Leukocyte Migration to the Central Nervous System.' Experimental Autoimmune Encephalomyelitis (EAE) is the mouse model of Multiple Sclerosis At the same time of EAE induction, Artesunate treatment was started for five consecutive days. Artesunate treatment reduced the clinical signs of EAE. Artesunate is an interesting candidate for MS amelioration as based off of animal studies it can reduce MS clinical severity but can also act against viral infections that can sometimes exacerbate Multiple Sclerosis. "Artesunate treatment reduced the clinical signs of EAE"
Human Herpes Virus 6 (HHV-6) and Epstein Barr VIrus (EBV) are the two most well studied viruses implicated in MS pathogenesis. These viruses appear to exacerbate disease in select individuals (See out MS and Viruses Posts!) Artesunate has been shown to reduce HHV-6 disease in case report describing a child with a widespread HHV-6 infection as well as in cell studies. The child did not respond to anti-viral treatment with the medication gangciclovir. Artesunate has in-vitro evidence against EBV. Artesunate has activity in HHV-6 and EBV, making an excellent candidate to deal with MS related viral infections but also mitigate inflammatory disease (as seen in the mouse study above). Our Naturopathic clinic uses immunosciences labs viral panels to measure a full spread of viral infections including HHV-6 and EBV. We have significant experience at my Naturopath clinic in Edmonton working with wormwood derivatives for Breast, Prostate and Colon Cancer. It is extremely well tolerated and somewhat reasonable in cost. The herbal form Artemesinin or the intravenous form as Artesunate are both suitable options. I propose combining Artesunate or Artemesinin (or a combination of both) with anti-viral medications such as Valganciclovir if MS related viral infections are found. Considering glycyrrhizic acid (licorice extract - oral and IV) and intravenous ozone are other anti-viral therapies that can be leaned on. Humic acid and Lauricidin are also low cost, easily administered oral therapies that can help non-specifically against a range of viruses (and hypothetically against HHV-6).
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Dr Muradov and Dr Deol operate TruMed Edmonton Naturopath Clinic TruMed has a special focus in IV Vitamin Therapies and Treatments We offer Edmonton a large range of Naturopathic IVs IV Vitamin C & IV Glutathione are most commonly used Vitamin Infusions for Fatigue and Mood are extremely popular Select references: J Clin Virol. 2013 Jun;57(2):157-60. First therapeutic use of Artesunate in treatment of human herpesvirus 6B myocarditis in a child. Hakacova N1, Klingel K, Kandolf R, Engdahl E, Fogdell-Hahn A, Higgins T.
|
Fampyra and MSFampyra and MS
Posted On January 28 2017 I reviewed a 2016 clinical trial evaluating fampridine (4-aminopyridine) the mobility improving pharmaceutical treatment. Fampridine prevents the release of potassium from demyelinated areas of neurons. Consequently, disrupted nerve conduction may be partly restored. Results across studies are supportive of fampridine yielding an increase in walking speed of approximately 25 %
This trial was apparently in fairly mobile patients, as the majority were able to complete a six minute walk. Often, MS patients undergo a single 25 foot walk test at neurologist offices. "Results across studies show an increase in walking speed of 25 %"
At my naturopath clinic in Edmonton we are often exploring ways to enhance walking, nerve function and fatigue in my MS patients. We offer a range of alternative testing and treatments for our MS patients. We have extensive experience with IV administration of various Vitamins and Glutathione for our Edmonton MS patients. Co-administration of B1(Thiamine) and Fampyra may have additive effects as both agents enhance nerve conduction. Other B-vitamins are likely helpful, B12 in particular which has synergy with B1 for nerve conduction and of course is needed for re-myelination after acute MS exacerbations. Furthermore, stretching, intramuscular botox and oral cannabinoids are other ways to improve mobility by reducing spasticity (see our Cannabinoid post)
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Dr Muradov oversees TruMed Edmonton Naturopath Clinic TruMed offers Edmonton comprehensive alternative testing We are commonly running Food Allergy Testing and Hormone testing for MS We also offer infectious testing: Viral testing & Candida yeast testing. Thyroid and adrenal testing is also helpful for our MS patients. Select references: BMC Neurol. 2015 Sep 24;15:171. doi: 10.1186/s12883-015-0431-0. Dynamic walking features and improved walking performance in multiple sclerosis patientstreated with fampridine (4-aminopyridine).
Keune PM1,2, Cocks AJ3, Young WR4, Burschka JM5, Hansen S6,7, Hofstadt-van Oy U8, Oschmann P9, Muenssinger J10.
|
Vitamin D and MS
Vitamin D and MS
Posted On February 12 2017 In January 2017 I attended a presentation by Reinhold Vieth - renowned Vitamin D researcher Full-skin exposure to Sunlight or UVB is equivalent to a 10,000 IU daily dose of vitamin D3. He discussed VitD4MS study where the vitamin D dose was escalated up to 40 000 IU daily and then back down over a 52 week period. VItamin D was given daily, orally with calcium, which in animal studies is needed for full D immune modulating activity. At a serum level of 250nmol/L the urinary calcium levels started to rise. Therefore it was suggested to use doses that result in serum levels just below this threshold. In 2012 a 96 week study found no change in the relapse rate of patients taking 20 000 IU per week (or about 3000 IU per day). The serum rates went from 50 to 121 nmol/L. Therefore, at the very least, there is an argument to be made for doses to definitely be higher than 3000 IU per day and higher blood levels than 120 nmol/L. "Full exposure to sunlight is equivalent to 10 000 IU of Vitamin D3"
A bit of math: We know that a 2000 IU dose results in serum levels of 50 nmol/L (or 1000 IU = 25nmol/L) Therefore, a dose of 9000 should result in levels around 225 nmol/L. This would avoid coming too close to 250 (which would would get from a repetitive dose of about 10 000 IU). - which is where urinary calcium starts to rise. Therefore, 9 000 IU per day will bring the blood levels to a point slightly below 250 and would avoid increases in urinary calcium. At my Edmonton Naturopathic clinic, we often suggest 8-10 000 IU doses with co-administration with calcium to better approximate the effects of this study.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: Mult Scler. 2012 Aug;18(8):1144-51. Feb 21. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Kampman MT1, Steffensen LH, Mellgren SI, Jørgensen L.
Rienhold VIeth (2017). Primary Prevention and therapeutic potentials in the contexts of Multiple Sclerosis and Prostate Cancer |
Viruses and MS Post 1
MS & Viruses Post 1
Posted On March 5 2017 I reviewed the 2017 Journal article "Multiple sclerosis: an example of pathogenic viral interaction?" Two viruses that have consistent evidence for their etiological role in MS are EBV and HHV-6. MS is thought traditionally to not have a known cause. I personally believe this may underlie exacerbations in a subset of MS patients. EBV persistence has been described in post-mortem brain tissue of MS patients The author suggests the basic concept is that HHV-6A actives latent EBV in MS lesions. I am a strong proponent of checking viral levels in MS patients with persistant inflammatory disease. I recently had a patient with a strong, multi-system MS relapse and we decided to check her EBV levels and she had an acute EBV infection at the same time as her relapse. This is a strong suggestion that in some patients these infections may strongly influence MS disease activity. I propose the use of Valganciclovir which has efficacy against both EBV and HHV-6. Treatment with valganciclovir may be long term, mimicking cytomegalavirus (CMV) prophylaxis regimines in transplant patients. Induction therapy using 900mg twice daily for three weeks followed by 900mg once daily for extended periods Case resports describe use from anywhere from 6 weeks to 18 months for HHV6 encephalitis, and 6 months in CFS patients with EBV and HHV6 elevations. However, the 6 month trial in CFS showed no changes in EBV or HHV6 IgG titres which suggests Valganciclovir may need support from additional anti-virals Therefore, I suggest that other anti-virals are used in tandem. As discussed in my Artesunate Post, wormwood derivatives might be a suitable add-on therapy with regards to HHV-6. "MS relapses may be related EBV and HHV-6 infection"
In summary, the viral theory in Multiple Sclerosis is an empowering theory. It allows us to take control of MS exacerbations by addressing the infections that may trigger them.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select references: Virol J. 2017 Feb 28;14(1):42. doi: 10.1186/s12985-017-0719-3. Multiple sclerosis: an example of pathogenic viral interaction? Fierz W1.
Share on Facebook |
MS and Viruses Post 2MS & Viruses Post 2
Posted On March 9 2017 I Reviewed the 2005 clinical trial from Höllsberg where MS patients were given the oral anti-viral medication valacyclovir. Epstein-Barr virus (EBV) and human herpesvirus 6B (HHV-6B) were analyzed in saliva and blood from MS patients in a randomized, double-blind, placebo-controlled treatment study using Valtrex (valacyclovir) EBV and HHV-6B DNAs were detected in 41% and 65% of saliva samples, and valacyclovir reduced the EBV significantly Blood contained EBV and HHV-6B DNAs in 17% and 25% of the samples, which was not reduced by valacyclovir Patients with high disease activity had a significantly higher frequency of EBV and HHV-6B - this correlation was found strongly in plasma "Patients with high disease activity should be screened for both EBV and HHV-6"
Valacyclovir appears to be only effective for EBV treatment which explains in part why only EBV reduction was seen in this trial. Based on a different trial (PMID: 17276366) I propose treatment with Valganciclovir also known as Valcyte.
However, one may consider co-administration with natural anti-virals such as Glycyrrhizic acid and Artemesinin to maximize the possibility of viral reduction (See Artesunate Post) as viral resistance can occur even with Valganciclovir.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. |
MS and Viruses Post 3MS & Viruses Post 3
Posted On May 1 2017 One could postulate that the intermittent reactivation of a virus might parallel the relapsing/remitting pattern seen commonly in relapsing forms of MS. Patients with high disease activity had a significantly higher frequency of EBV and HHV-6B.
The treatment was given at a high dose for a period of two years. "HHV-6 as the etiologic agent . . . but this may not be the case for all MS patients."
Valacyclovir appears to be only effective for EBV treatment which explains in part why only EBV reduction was seen in this trial. Based on a different trial (PMID: 17276366) I suggest treatment with Valganciclovir also known as Valcyte.
However, I do suggest co-administration with natural anti-virals such as Glycyrrhizic acid and Artesunate to maximize the possibility of viral reduction (See Artesunate Post) as viral resistance can occur even with Valganciclovir.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Mult Scler. 2005 Jun;11(3):286-95 A randomized clinical trial of valacyclovir in multiple sclerosis Friedman and friends. |
MS and Viruses Post 4MS & Viruses Post 4 Posted On Feb 2018 As mentioned already, two viruses that today remain the most strongly associated with MS are the herpesviruses Epstein–Barr virus (EBV) and human herpes virus 6 (HHV-6).
Also, varicella zoster virus (VZV), cytomegalovirus (CMV), measles, rubella, mumps and herpes simplex virus (HSV-1) have been implicated in MS.
There are two species of HHV-6, HHV-6A and HHV-6B.
Eleven patients had relapsing/remitting MS and 15 had secondary progressive MS.
"Risk of exacerbation was 2.5x higher for the patients with HHV-6 reactivation"
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Viruses in chronic progressive neurologic Viruses and endogenous retroviruses in multiple sclerosis: Anti-Human Herpesvirus 6A/B IgG Correlates with Relapses and Progression in Multiple Sclerosis J Med Virol. 2003 Jan;69(1):111-7. |
MS and Viruses 5MS & Viruses Post 5 Posted On Jan 2020 Humans are the primary hosts for 8 known species of herpesviruses (HSs): herpes simplex virus-1 (HSV-1), herpes simplex virus-2 (HSV-2), varicella-zoster virus (VZV or HHV-3), Epstein-Barr virus (EBV or HHV-4), cytomegalovirus (CMV or HHV-5), human herpesvirus 6 (HHV-6), human herpesvirus 7 (HHV-7). 141 blood samples from MS patients were collected in North-eastern Poland during 2017 with no relapse at the time of blood sampling - 36.2% males and 63.8% females. The group had an average age of 41 with the disease for about 8 years. The authors found that MS patients were significantly more likely to be positive for anti-EBV IgG and anti-HHV-6 IgG compared to controls. No significant difference was found in the presence of antibodies against HSV-1,2, CMV and VZV in both groups. Also, in MS patients, anti-CMV IgG was significantly higher in females. The authors found that MS patients were significantly more likely to be positive for anti-EBV IgG and anti-HHV-6 IgG compared to controls.
86.49% of the patients were positive for both anti-HHV-6 IgG and anti-EBV IgG. The role of CMV in MS is currently controversial and is still being researched. In the study, no significant difference in the CMV prevalence between the MS patients and the healthy individuals in the control group. Paradoxically, reports of a better disease outcome in seropositive CMV patients has been reported. This study revealed a very high percentage of MS patients who were seropositive for anti-HHV-6 IgG (92.91%). In a Finland study, HHV-6 antibodies were found in 100% of the serum of patients with MS and only in 69.2% of the serum of the controls. In a Latvian study, 61.5% of patients were seropositive in the MS group for HHV-6 and only 29% in the group of patients with other neurological disorders. Some findings that additionally support the role of HHV-6 in MS as increased HHV-6 DNA was found in MS plaques. As mentioned already, two viruses that today remain the most strongly associated with MS are the herpesviruses Epstein–Barr virus (EBV) and human herpes virus 6 (HHV-6) and what our Naturopathic doctors see in our Edmonton MS patients mirrors these studies - that is, HHV-6 and EBV are found frequently in our MS patients, This study looks at IgG - more of an indication of previous exposure vs IgM. Our Naturopaths always run IgG and IgM and if there is a substantial IgG or IgM elevation we always initiate anti-viral treatment.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Dr Muradov oversees TruMed, an Edmonton Naturopath Clinic. |
MS and CannabinoidsKetosis and MS
Posted On April 11 2017 MS is a multi-front battle: reducing relapses is only part of the fight. Offsetting long term neurological declination is an area that has not been fruitful from a pharmaceutical standpoint. However, nutrients and diet are probably the most promising interventions to impact the health of battered brain cells. At my Naturopath clinic in Edmonton we often discuss different dietary strategies to impact neurodegeneration. I discussed this topic at length in an article I wrote for IHP magazine in 2013. However, at the time of writing in 2013 the information on ketogenic diets and Multiple Sclerosis was scant. Ketosis has had a rebirth lately and the strongest evidence for MS came out in June 2016. As an Edmonton naturopath with a special interest in MS and Cancer, this dietary approach has relevance to both conditions. The portion of a brain cell that connects to other brain cells is called an axon (the axons are where demyelination occurs). The axons that survive demyelination contain have a much increased energy demand. Glucose or sugar, is typically the primary fuel for most of the cells around the body. However, it appears that damaged axons have resistance to glucose entry. By inducing a carbohydrate restricted state, the body can shift into burning fats as a primary fuel instead of glucose. When this happens the body is said to be in a state of Ketosis, that is, its it burning a fat by-product called a ketone and these ketones replace glucose as the brain's main sources of fuel A ketogenic diet has a favourable effect on mitochondrial function: it reduces levels of reactive oxygen species (a damaging by-product of energy production) and increases ATP creation. Furthermore, a ketogenic diet raises glutathione levels - the body's master anti-oxidant (through the Nrf2 pathway). FInally, ketones help preserve mitochondrial function. The first trial to evaluate ketosis in human MS patients was released much to my delight in June 2016. The authors first discussed a fasting diet where 3 of 7 days per week were spent fasting in mice. Interestingly, this diet caused a 40-50% reduction in circulating immune cells thereby reducing the total number of auto-reactive T-cells. Furthemore, the diet seems to promote differentiation of OPCs (Oligodendrocyte precursor cells - the cells that myelinate axons) and hastened myelination. In the human trial, 60 patients were randomly assigned: - 20 consuming a control diet - 20 on a Ketogenic diet - 20 on a mediterranean diet (after a 7 day fast). Ketogenic diet had less than 50 grams of carbohydrates. Whereas, the control diet had patients just continuing their standard diet. The mediterranean group consisted of no more than 350 calories per day as soup broth for the first seven days before continuing on a mediterranean diet for 6 months. The mediterranean diet is a commonly used Naturopathic strategy for improving cardiovascular health with fruits, vegetables, and olive oil. After 6 months, the control diet had a slight declination in physical function whereas the ketogenic groups has substantial improvements in physical function and fatigue. The mediterranean group had reasonable improvements in mental health but physical function and energy were marginally better. During the 6-month study period a total of 8 relapses occured: 4 in the control diet, 1 in the ketogenic group and 3 in the mediterranean group. Ultimately, this small study shows proof of concept using ketogenic diets in MS patients with beneficial effects on physical function, energy and perhaps relapse frequency! Interestingly, Ketogenic diets are now being discussed for traumatic brain injury and spinal cord injury as animal studies demonstrate improved rehabilitation outcomes if animals are ketogenic. Most interestingly, in brain injuries demyelination is part of the process and improved outcomes from these injuries suggests that ketosis may improve re-myelination. I work with ketogenic diets with my MS patients at my Naturopath clinic in Edmonton. I have seen some functional improvements overtime and improved energy similar to what was seen in the trial above. The book "The Wahls Protocol" is a good resource for patients looking for guidance around structuring a ketogenic diet. Of course, a diet where fat is the main fuel will take a lot of focus and preparation so it's not for everyone. Overall, however, the ketogenic diet is more of a long term move to preserve neurological function and offset deterioration and prevent entry into progressive MS. "After 6 months the ketogenic group had substantial improvements in physical function and fatigue."
Ultimately, using a ketogenic diet even intermittently may help preserve neurological function by providing improved energy production in demyelinated nerve cells. Burning ketones result in greater ATP (energy) production in nerve cells as demyelinated axons have poorer absorption and utilization of glucose. Evidence from a 2016 trial suggested that Ketogenic diets in MS patients seem to enhance physical function and energy and may even serve to reduce relapse frequency. Hopefully this will spur a longer trial to see how well this type of diet can offset neurodegeneration in MS patients in the long run. Until then, I will continue to recommend Ketogenic diets at my Edmonton Naturopath clinic as well as nerve preserving supplements to maintain neurological function in my MS patients.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Dr Muradov oversees TruMed, an Edmonton Naturopath Clinic. Share on Facebook |
Seroquel and MSSleep, Seroquel and MSPosted On April 21 2017 The most common symptom that I see in my MS patients at my Edmonton Naturopath clinic is insomnia. Sleep disturbance is reported by up to 50% of patients with MS; it certainly contributes to the fatigue that is commonly associated with the disease and may exacerbate many other symptoms of MS. The most common medication prescribed by neurologists or GPs is zopiclone, a bitter tasting yet effective sleep medication. However, it often loses efficacy with time, is greatly addictive (despite the claims that it isn't) and often requires upwards dose escalation. The monograph for Zopiclone suggests use for 7-10 days which further demonstrates it's not meant for chronic sleep issues present in most MS patients. In a previous post I discussed CBD from Cannabis as a naturopathic anti-spasmodic, anti-inflammatory and sleep inducing extract. So this can certainly be considered as a first line sleep treatment especially if there is concurrent inflammation and muscle spasticity. Quetiapine fumarate (Seroquel) is an atypical antipsychotic medication with mood boosting, sleep inducing and remyelinating properties and it's low cost and highly effective for sleep induction. Before reading this article, it is important to realize that there is only a very small list of agents that can enhance remyelination in experimental models, so this information needs to be taken extremely seriously. The evidence for Quetiapine was collected and summarized by Zhornitsky and colleagues in an excellent 2013 article from the University of Calgary. This blog post is based primarily off of the information they gathered. Recent studies revealed that Quetiapine possesses potent remyelinating and neuroprotective In "EAE" - the MS animal model, Quetiapine seemed to reduce activated immune cells, scavenged free radicals (anti-oxidant) while at the same time increasing the maturation of oligodendrocytes (which are responsible for myelination in the brain). So it has a dual, anti-inflammatory and nerve revitalizing action. Amazing. The anti-oxidant actions seem to be related to upregulated SOD activity - a principle anti-oxidant enzyme system in the body. Also, some of the pro-nerve activity of Quetiapine may in part due to increased levels of BDNF (brain derived neurotrophic factor) - which plays a role in remyelination and neuroregeneration. I discussed increased BDNF levels from my previous post on the medication Lemtrada/Alemtuzumab therefore it appears that the use of Lemtrada and Quetiapine may have synergistic neurorepairative actions. FInally, Quetiapine appears to down-regulate most of the pro-inflammatory cytokines that are common in MS in animals and humans. The authors note that several large, placebo-controlled trials have shown that the efficacy of Quetiapine exceeds that of some established antidepressants for depression and for anxiety. Therefore, it can help balance mood issues if they are present. Quetiapine immediate and extended release possess strong sedative properties, and they have a relatively short half-life so they it can be administered at bedtime without producing significant next-day drowsiness. From what I've seen in my patients, if there is a bit of next day grogginess it quickly passes as patients become accustomed to it. If falling asleep is the predominant issue then immediate release is the more appropriate preparation for you to ask for from your health care provider. In terms of side effects, weight gain, changes in blood cholesterol and dry mouth, nausea and headaches are all possible. However, slow-titration to the recommended doses and/or using the slow-release preparation will greatly reduce incidence of the latter three side effects. Translating animal to human doses, the authors recommend about 300mg of Quetiapine. I find that the sedative effects are usually occuring at doses less than 100mg, therefore, we increase to sleep inducing doses within a few weeks (using 25mg tabs) before continuing to the recommended 300mg dose over 3-6 months. Lastly, you may be wondering why a Naturopathic Doctor is recommended a prescription anti-psychotic medication. When we summarize the effects: promyelinating, sleep inducing, anti-inflammatory and mood elevating, I don't know of a natural substance that can claim such a wide spread of pro-MS effects. At the end of the day, I practice integrative medicine which is about combining the best of standard and alternative options. Low dose naltrexone is another pharmaceutical option that I use at my Naturopath clinic in Edmonton for both MS and Cancer (see my LDN post!). "Quetiapine reduced pro-inflammatory cytokines and increased myelination" In summary, there are few options that are as promising at Quetiapine for not only symptoms common to MS (insomnia, depression/anxiety) but as a long term neuroprotective/neurorestorative treatment.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Dr Muradov runs TruMed Naturopath Clinic in Edmonton, AB Select References: CNS Neurosci Ther. 2013 Oct;19(10):737-44. Quetiapine fumarate for the treatment of multiple sclerosis: focus on myelin repair. Zhornitsky S1, Wee Yong V, Koch MW, Mackie A, Potvin S, Patten SB, Metz LM.
|
Lithium and MSLithium and MSPosted On April 30 2017 Lithium is a mineral with properties that can be advantageous for MS patients. Mood stabilizing and neuroprotective capabilities make it an attractive, low cost option to add to a MS patient's armament. I make use of low dose Lithium orotate in my Naturopathic practice in Edmonton. It has excellent tolerability and is a low cost intervention that is an easy addition for most of my patients. Lithium's neuroprotective activity are from its ability to inhibit an enzyme called glycogen synthase kinase-3 (GSK3). GSK3 inhibition protects neurons from a wide variety of insults. One of GSK3’s functions is to facilitate signals that cause neurons to die. Therefore, its inhibition may improve retention of neurons which has benefit not only in MS but other neurodegenerative conditions like Alzheimer's and Parkinson's. GSK3 is activated when neurons are stressed. Lithium can bolster the resilience of neurons by inhibiting GSK3 to put a brake on the deleterious effects of stress and toxic substances. Transcription factors regulate the expression of genes. GSK3 inhibition by Lithium allows for activation of the transcription factor Nrf2, which reduces inflammation and improves anti-oxidant function. Curcumin, Green Tea, Exercise, Fish Oil/DHA and Caloric restriction also activate Nrf2. Other well-documented Lithium outcomes include axonal regeneration, improved mitochondrial function, re-myelination, and the generation of new neurons. So its relevance to MS is extremely clear. A 2008 study looking at the animal model of MS (EAE) and Lithium. Pre-treatment with Lithium markedly suppressed the clinical symptoms of EAE induced in mice: it greatly reduced demyelination and white blood cell infiltration in the spinal cord. Lithium administered after disease onset, reduced disease severity and facilitated partial recovery. Therefore, Lithium appears to have both a disease mitigating action as well as a neuroprotective action to hasten recovery from relapses. I'm suggesting use of Lithium Orotate. Lithium carbonate is used in Bipolar disorder and is dosed between 900 – 1800 mg daily, and is available by prescription only and requires monitoring of blood levels, kidney and thyroid function. Lithium orotate is a nutritional supplement and comes in 5mg tablets, usually dosed no more than 20mg daily and does not require routine monitoring. I use it amongst other naturopath neuroprotective strategies such as: green tea extract, fish oil, vinpocetine, ginkgo, B-vitamins, acetyl-L-carnitine, Glutathione and PQQ. Interestingly, Lithium can inhibit various viruses therefore it may have an indirect role affecting MS disease activity if a patients disease is propagated from Viral infections (See MS and Viruses Post 1). In my Edmonton practice, I often suggest testing for a broad range of Viruses for actively relapsing MS patients to see if this is an area we can impact. From what I've seen in my Naturopathic practice, the MS population is susceptible to mood issues secondary to neurological dysfunction inherent to the disease process. The mood stabilizing effects may be beneficial if a MS patient has mood or depression. Interestingly, 7-UP Soda previously contained Lithium; apparently responsible for the "Up" portion of the name. "Lithium reduces inflammation and increases myelination"
In summary, lithium is an exciting mineral with a large spread of neuroprotective properties. Axonal regeneration, improved mitochondrial function, re-myelination, and the generation of new neurons are potential actions. Furthermore, reduction of inflammatory disease severity is also a possibility from Lithium.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select References: Cerebrum. 2016 Feb 1;2016. pii: cer-02-16.
Lithium to the Rescue. Jope RS, Nemeroff CB. |
Mesenchymal Stem Cells and MSMesenchymal Stem Cells
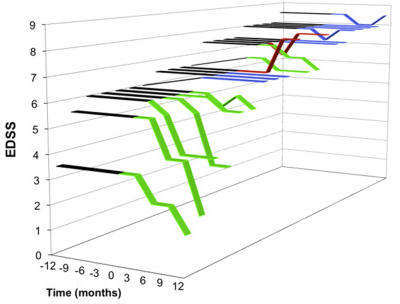
Posted On February 12 2018 Mesenchymal stem cells (MSCs) MSCs have immunomodulatory and repair-promoting effects (released trophic factors) and ability to traffic to areas of tissue inflammation and damage and are most commonly derived from bone marrow, umbilical cords or adipose (fat). The first few studies described below use bone marrow derived MSCs which can be obtained readily and safely from adult bone marrow, and autologous (from self) MSCs can be administered safely without the need for immunosuppressive treatment to prevent rejection. Adult MSCs were shown to be less prone to genetic abnormalities and malignant transformation thus implying a low risk for treatment-related malignant neoplasms. Autologous cells reduce potential concerns with transmitting infection or cancer from the donor. Some studies indicate repeated administration stimulates rejection which is an issue if multi-dosing is planned (16118325). Adipose-derived stem cells (ASCs) are adult mesenchymal stem cells (MSCs) that reside in fat that can also be used in an autologous manner. Human Umbilical Cord Stem Cells (hUC-MSCs) have compared to BM-MSCs, have higher proliferation and differentiation abilities, and stronger immune tolerance and can be used in an allogenic manner. This article will also discuss both intravenous (IV) and intrathecal (IT - into the spinal canal) administration on MSCs. A possible drawback of the intravenous administration of MSCs is that most of the cells injected into the blood will home to the lungs, lymph nodes, and other tissues, greatly reducing the number of cells available to migrate to the CNS. Whereas, intrathecal delivery of cells may focus their effects directly on the CNS, without producing systemic effects. 1 million cells per kg of body weight is suggested by multiple sources to be optimal for effective immunomodulation as an IV dose. Bone Marrow Stem Cells (BM-MSCs) An uncontrolled study by Bonab et al. in secondary progressive multiple sclerosis (SPMS) demonstrated modest results from a single spinal injection of MCSs (29.5 million cells) after 12 months observation. Six months after the injection, 72.8% of patients (16/22) revealed stability in the course of the disease, while EDSS improved in 3 patients and deteriorated in another 3 patients. Clinical course of the disease (measured by EDSS) improved in 4 (0.5 points) , deteriorated in 6 and had no change in 12 patients. In MRI evaluation, 15 patients showed no change (halting of progression) whereas 6 patients showed new T2 or gadolinium enhanced lesions (1 lost to follow-up). The authors concluded "that MSC therapy can improve/stabilize the course of the disease in progressive MS in the first year after injection with no serious adverse effects. Repeating the study with a larger sample size, booster injections and longer follow-up using a controlled study design is advised." The same group had a previous trial: a mean number of 8.73 million cells were administered intrathecally (spinal) to 10 patients with progressive disease; all had progressive disease that had not responded to disease modifying agents. During 13 to 26 months of follow up the EDSS of one patient improved from 5 to 2.5 score. Four patients showed no change in EDSS. Five patients' EDSS increased. Another group treated 7 SPMS patients with intrathecal injection of 47 million cells (5 times the previous study). In a trial by Rice (2010) "SIAMMS trial" six patients with starting EDSS of 6.0 were treated with IV autologous whole bone marrow preparation, which included BM-MSCs (1.1 million cells/kg). Five of the patients showed no signs of disease progression (EDSS unchanged). Improvements were seen in all components of the MS Functional Composite Scale at 1 year, but these were not statistically significant. (For example, in the timed 25-ft walk, the mean time taken improved by 0.19s). Connick (2012) used cryopreserved BM-MSCs in 10 Secondary Progressive MS-Patients (1.6 million cells/kg). There was minor reduction after treatment in general disability progression measured by EDSS but the study did not identify a change in the Multiple Sclerosis Functional Composite or in measures of depression, cognition, and self-reported effect of MS on daily living. However, there were improvements in visual acuity. In terms of safety, one group (Harris et al) investigated the use of neural progenitors derived from bone marrow MSCs ("MSC-NPs") in 6 progressive MS patients over a period of 7.4 years. These are said to have properties that are theorized to minimize the risk of ectopic differentiation after CNS transplantation. These patients were refractory to all conventional MS treatments at the time of inclusion and had an average EDSS of 6.5. These were also given intrathecally directly into the spine and patients received between two and five treatments. Overall the effects were subtle. None of the patients benefited after the first injection. Mild EDSS benefits were seen after multiple injections, typically a .5 point improvement. However, this is in contrast to the steady decline they experienced prior to the study. All six patients had been followed for a mean of 7.4 years post-initial injection with a minimum of more than 6 years post-treatment. There were no serious adverse events reported and no evidence of cancer or abnormal tissue growth. In 2018, a follow up study was done by Harris: MSC-NPs were given to 20 patients with progressive MS (PPMS and SPMS). MSC-NPs were administered intrathecally in three separate doses of up to 10 million cells per dose, spaced three months apart. The study cohort had a mean EDSS of 6.8 (range 3.5 to 8.5) and mean disease duration of 18.8 years. Only six of the twenty patients were male and 80% of the patients had SPMS. Of the 20 study subjects, 15 (or 75%) demonstrated neurological improvement associated with MSC-NP treatment. Muscle strength testing showed that 14 of the 20 subjects (70%) showed objective increase in muscle strength. Most of the subjects (13 out of 14) demonstrated improvement in lower limb strength. Four out of the ten ambulatory subjects showed a greater than 20% improvement in timed twenty five foot walk speed compared to pre-treatment. Overall, there was a decrease in median EDSS from 6.8 at baseline to 6.5 six months post-third treatment. Eight study subjects (40%) demonstrated at least a 0.5 point improvement in EDSS six months post-treatment, with four of the eight subjects showing an improvement of 2.0 or greater positive change compared to baseline. Ten study subjects showed continued stable EDSS throughout the course of treatment, and two study subjects had evidence of disease progression as determined by a decline in EDSS.
In a 2010 Israeli study (Karussis et al) fifteen patients with MS (EDSS 6.7) and 19 with ALS were enrolled and given intrathecal bone marrow derived MSCs. 14 Fourteen patients (5 with MS and 9 with ALS) also received intravenous MSCs. The type of MS was not specified beyond "intractable MS," that is, unresponsive to previous treatments. MS patients were given 63 million cells intrathecally and 25 million intravenously. In patients with MS, the mean EDSS score declined gradually from 6.7 before the treatment to 6.1 at 1 month, 5.9 at 3 and 6 months after MSC injection. More specifically, at the end of the 6 months of follow-up: EDSS reduced by: 0.5 in 5 patients, 1.0 in 1 patient, 1.5 in 3 patients, 2 degrees in 1, and 2.5 degrees in 1 No adverse effects were seen up to 25 months of observation. In a 2014 paper by Llufriu, looked at patients with RRMS or SPMS with EDSS between 3.0 and 6.5 which is dramatically lower than some of the previous studies. 5 patients were randomized to receive IV BM-MSCs and three recieved placebo. At 6 months, patients treated with MSCs had a trend to lower mean cumulative number of gadolinium enhancing lesions on MRI and TH1 cells in the blood. No significant treatment differences were detected in the secondary endpoints. A 2017 paper by Cohen et al. looked at patients with RRMS or SPMS with EDSS between 3.0 and 6.5 as well. 24 patients received a single IV infusion of 1–2 × 106/kg body weight BM-MSCs. Minor improvement in the EDSS reflected improvements in ambulation (walking), and cerebral and sensory subsystems. Mean EDSS dropped by about .2-.3. The authors noted "exploratory efficacy measures demonstrated no substantial evidence of inhibition of disease activity, tissue repair, or recovery of function comparing 2–3 months pre-infusion to 6 months post-infusion." Paradoxically, in contrast with Llufrui, the Gadolinium enhancing lesions on MRI appeared to mildly increase. However, the authors were concerned that the cryopreserved cells thawed just before infusion could impair their activity. A 2017 by Dahbour looked at a total of 10 patients that completed post treatment follow‐up analysis. The studied patients were 4 females and 6 males, 8 of them had SPMS, and 2 had RRMS. This trial looked at high dose intrathecal BM-MSCs as well as the Mesenchymal Stem Cell Conditioned Media which is reported to have immune‐modulating soluble cytokines, growth factors, and brain repair molecules in the MSC‐CM. BM‐MSCs were injected intrathecally into patients immediately after harvest (100 million cells) - to limit cell death due to cryopreservation. After a month 18 mL of MSC‐CM corresponding to each patient's MSCs was given intrathecally as well. Of the 10 patients, 4 corresponding to 40% showed no change in their EDSS, another 40% of patients deteriorated while 20% improved with average improvement in the Timed 25‐Foot Walk (1.1 seconds faster). Two patients had a remarkable decrease in their EDSS. Both were SPMS, the first patient presented with an EDSS of 5.5 which became 2 at twelve months, while the other patient went from a score of 6 to 1.5 Summary: The best BM-MSC results were found in Karussis which suggests that both IV and IT delivery may yield the best overall MSC results. However, Dahbour's study suggests that a very large intrathecal dose might be helpful as well. Umbilical Cord Stem Cells (hUC-MSCs) Umbilical cord stem cells were first looked at in MS in 2009: a patient with rapidly progressing refractory progressive MS was given IV and intrathecal hUC-MSCs. The disease course was stabilized with signs of improved sensory function and muscle strength. After 5 months, the EDSS score was down to 5.5 from 8.5 at study entry. In a 2014 trial, out of twenty-three patients, 13 of them were given hUC-MSC therapy at the same time as anti-inflammatory treatment, whereas the control patients received the anti-inflammatory treatment only. The experimental group had either RRMS or SPMS with mean disease duration of 2.90 years and EDSS score of 6.98±1.2 (range 4.0–8.0) indicating patients with a rapid disease course and somewhat significant deterioration. The serum levels of the Th2 cytokines IL-4 and IL-10 increased significantly over the first 3 months after treatment in patients in the experimental group compared to those in the control group. The serum levels of Th1/Th17 cytokines TNF-a and IL-17, however, significantly decreased in the first 3 months after treatment in the patients of the experimental group The EDSS scores at the end of the 1-year period were only slightly lower than that of the baseline in the experimental group (about 0.5 point decrease) the treatment apparently prevented, in the short term, the continuous deterioration the patients were experiencing. Case report of a 25 year old that after having a third relapse within three years affecting his walking received at first three infusions of BM-MSCs (IV and intrathecal) and then hUC-MSCs (IV) and then BM-MSCs (intrathecal) and then finally one last infusion of hUC-MSCs (IV). Throughout the 4 year treatment period the patient remained completely free of clinical and radiological disease activity with no treatment other than BM and hUC-MSC. He made a good recovery from the severe relapse in 2008 and remains able to walk unaided for > 500 meters. Adipose Stem Cells (ASCs) Adipose Stem Cells (ASCs) were studied in a 2016 trial by Stepien where 20 MS patients were studied (13 RR and 7 SPMS). The baseline EDSS scores ranged from 3 to 6.5 (mean 4.6) in the first group and from 3.5 to 9 (mean 5.6) in the second group. The average disease duration was 9.5 years in the first group and 15.6 in the second group. All patients clinically deteriorated during the year preceding the study, with at least a 1-point increase in EDSS score. ASCs were injected intrathecally (4 million cells/dose) at the time of enrollment, and the injection was repeated at the 3rd and 6th months using cryopreserved ASCs. At the 18-month follow-up, EDSS scores had not changed in any of the patients from either group. Within 18 months of follow-up, 3 patients from the RRMS group had relapses without exhibiting progression in disability from baseline, and 7 patients showed “enticing” improvement on some of the exploratory efficacy measures however this was not elaborated on. It is likely the doses injected were too low. Placenta Stem Cells Placental Stem Cells are studied in a 2014 Study by Lublin. PDA-001, a preparation of mesenchymal-like cells derived from full-term human placenta. Ten patients with RRMS and 6 with SP-MS were randomly assigned to treatment: 6 to low-dose PDA-001, 6 to high-dose PDA-001, and 4 to placebo. EDSS scores over the 1-year follow-up period were similar to baseline or were improved for the majority of patients who received PDA-001, which hints at a potential reparative effect in this disease. "IV and IT administration appears to yield the best results"
In summary, stem cells have been studied from Bone Marrow (BM-MSCs), Umbilical Cord (hUC-MSCs), Adipose (ASCs) and Placenta in MS. MSCs have immunomodulatory and repair-promoting effects. It appears that combining both the IV and IT (intraveous and intrathecal) routes has the highest potential for large scale, clinically meaningful EDSS changes. However, repeated or large volume intrathecal injections also are very promising.
Dr. Eric Muradov
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Select References: Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study.
Bonab et al.
Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Harris et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis.
Karussis et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study.
Connick et al.
Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy
Dabour et al.
Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis
Rice et al.
Clinical Application of Autologous Adipose Stem Cells in Patients with Multiple Sclerosis: Preliminary Results
Stepien et al.
Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis
Cohen et al.
The Potential of Human Umbilical Cord-Derived Mesenchymal Stem Cells as a Novel Cellular Therapy for Multiple Sclerosis
Feng Li et al. Harris VK et al. |
Whole Body Vibration and MS
MS & Vibration
Posted on October 1 2017 In the last decade, whole body vibration (WBV) training has been increasing in popularity. In WBV individuals perform their exercises while standing on a vertically vibrating platform. Involuntary muscle contractions reduce the recruitment threshold for the muscles resulting in a more rapid activation of muscle fibers. At my naturopathic clinic we are often exploring options for improving spasticity in my MS patients. I've found a mix of cannabinoids, magnesium, exercise and frequent stretching fairly effective for mild to moderate spasticity. WBV falls under the umbrella of exercise with regards to spasticity. Stronger pharmaceutical options can also be helpful, most commonly injections of Botox into affected musculature as well as a medication named Baclofen. I Reviewed the 2013 Journal Article 'Effects of whole-body vibration training on physical function in patients with Multiple Sclerosis.' The authors set out to see if three-weeks of whole body vibration training in addition to a standard rehabilitation program improves walking ability. Forty-seven patients were assigned to the intervention group and underwent the whole body vibration training. Thirty-seven patients were assigned to the control group and were instructed to complete the same exercise as the patients in the intervention group without turning the vibration plate on. Overall average EDSS scores were 3.3 (corresponding to a level between light and moderate impairment) Sixty patients were included in the final analysis. First, patients in the intervention group completed a warm-up exercise consisting of three series of 30-second squats on the vibration platform with 30-second rest periods between squats. Subsequently, patients in the intervention group performed three series of 60-second squats while standing on the vibration platform. The training intensity was increased throughout the three-week training period by reducing the rest period between the 60-second sets. The control group did the same quantity of squats without the vibration activated. "Patients in the vibration group walked 14.7% further"
Patients in both groups showed significant improvements in their walking ability. In a separate article a group of MS patients received whole body vibration plus exercise three times per week, two weeks of no intervention and then four weeks of exercise alone three times per week. There was a greater reduction in muscle tone score spasticity using whole body vibration and exercise compared to exercise alone. As a naturopath doctor, I would recommend WBV as an adjunct to a comprehensive exercise and stretching program to enhance muscular strength, endurance and gait. The use of cannabinoids as well as Botox injections or Baclofen could be synergistic with this.
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Dr Muradov oversees TruMed Naturopath Clinic in Edmonton. TruMed Focuses on Hormones and Digestion We offer Edmonton advanced Hormone, Thyroid and Adrenal Testing TruMed has a special interest in Cancer and MS We offer integrative strategies for MS management for Edmontonians. We intermix naturopathic and standard medical strategies. Select references: NeuroRehabilitation. 2013;32(3):655-63.
Effects of whole-body vibration training on physical function in patients with multiple sclerosis.
Hilgers C1, Mündermann A, Riehle H, Dettmers C.
|
Testosterone and MS
MS & Testosterone
Posted on October 7 2017 I reviewed "Neuroprotective effects of testosterone treatment in men with multiple sclerosis," a 2014 clinical trial. MS relapses are associated with inflammation and the development of white matter lesion Interestingly, inflammatory activity and white matter lesion burden are only weakly correlated with clinical disease progression. Gray matter (GM) atrophy correlates strongly with clinical disability. Conversely, neuronal cell death and synaptic loss have been observed in gray matter lesions in MS. GM atrophy has been suggested as a surrogate marker for disease progression and neurodegeneration in MS Testosterone has been shown to be neuroprotective in animal studies The patients were observed prior to treatment for 6 months (observation phase), followed by a 12-month period of treatment with testosterone Participants were eligible if they met the criteria for clinically definite relapsing–remitting MS, had had at least one clinical relapse or the appearance of at least one enhancing lesion on MRI over the preceding two years, but were not receiving disease-modifying treatment. Avergae Expanded Disability Status Scale (EDSS) score of 2.0, disease duration of 12.5 years and age of 46 years. EDSS of 2.0 indicating a relatively benign clinical course further demonstrated by subjects choosing to not take DMTs. 10 g of gel containing 100 mg of testosterone (Androgel) applied topically daily for one year. During the observation phase (no treatment) GM loss was seen across the entire brain In the protection phase, essentially no further atrophy occurred. Instead, a significant increase in GM was observed in certain areas. "In the protection phase, essentially no further atrophy occurred."
The annualized gray matter atrophy rate in these untreated MS patients was -2.87% Compared to %.18 during testosterone treatment and reversed in some areas. This is the first report of a treatment-induced GM volume increase in MS. Testosterone's effects were thought to be neuroprotective rather than anti-inflammatory: neurogenesis & synaptogenesis.
Naturopath-Edmonton.ca Dr Muradov works at TruMed Naturopath Clinic in Edmonton. TruMed Focuses on Hormone Testing We offer Edmonton advanced Hormone, Thyroid and Adrenal Testing In-depth Saliva and Blood testing helps us understand where concerns originate. TruMed has a special interest in MS We offer integrative strategies for MS management for Edmontonians. We intermix naturopathic and standard medical strategies. Advanced Hormonal Testing and Treatments in Edmonton Select references: |
Borage Oil and MS
Borage Oil
Posted on October 17 2017 Gamma linoleic acid (GLA) is produced in the body as an intermediate in the metabolism of linoleic acid. "Relapse Rate was .35 in the High Dose Group"
Dr. Muradov is a Naturopathic doctor in Edmonton with a special interest in MS. Dr Muradov oversees TruMed Naturopath Clinic in Edmonton. TruMed has a special interest in Cancer and MS We also treat a range of concerns naturally. We offer integrative strategies for MS management for Edmontonians. We intermix naturopathic and standard medical strategies. Select references: Br J Nutr. 2007 Oct;98 Suppl 1:S46-53. Polyunsaturated fatty acids in the pathogenesis and treatment of multiple sclerosis. Harbige LS1, Sharief MK.
|